The system platform “Clinical Trial Quality Risk Monitoring
Method, Device, Computer equipment and Storage medium” in-house developed
by Tigermed group, was recently awarded the Invention Patent Certificate
authorized by the State Intellectual Property Office, realizing another
important breakthrough in indigenous R&D technology and innovation-driven
strategy.
Since the U.S. Food and Drug Administration took the lead in
recommending the adoption of risk-based monitoring (RBM) in 2013,
regulatory agencies in various countries have also revolutionized the
monitoring approach, advocating the adoption of RBM or more comprehensive
risk-based quality management (RBQM), and China has also released a new
version of GCP in 2020 and mentioned RBQM (Article 31), clearly proposing the
adoption of a systematic, risk-based approach to clinical trial monitoring.
China also released a new version of GCP in 2020 and mentioned RBQM (Article
31), clearly proposing to adopt a systematic, risk-based approach to clinical
trial monitoring.
One of the major benefits of using RBQM
is that the system can directly contribute to the quality, efficiency, and the
speed of clinical trials.
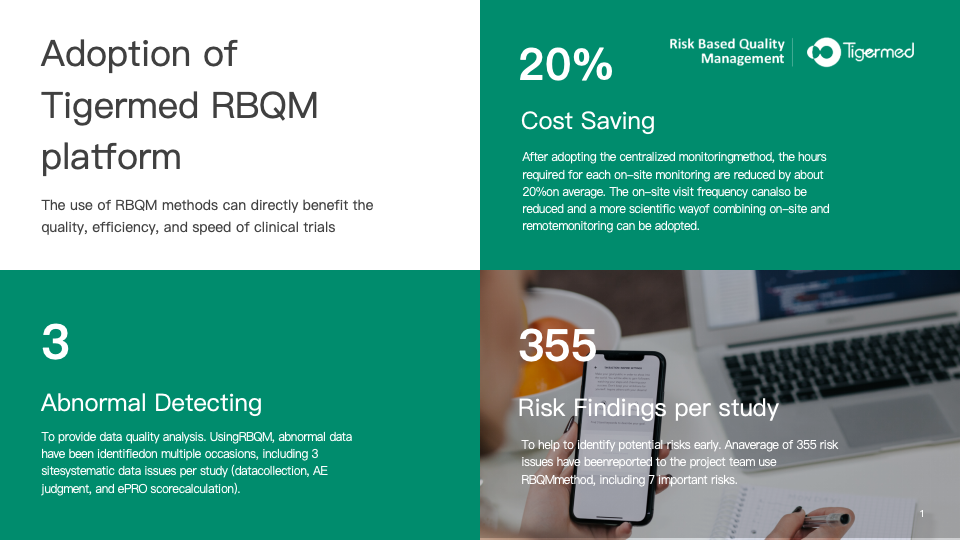
Since the adoption of the centralized
monitoring approach, the hours required for each on-site monitoring have been
consistently reduced by an average of 20%. According to Tigermed’s practices and
other survey findings, RBQM has consistently shown that cost savings can be
ultimately achieved by early detection of data anomalies, shortened data
management cycles, accelerated database locking, reduced lag in subject visit
data entry.
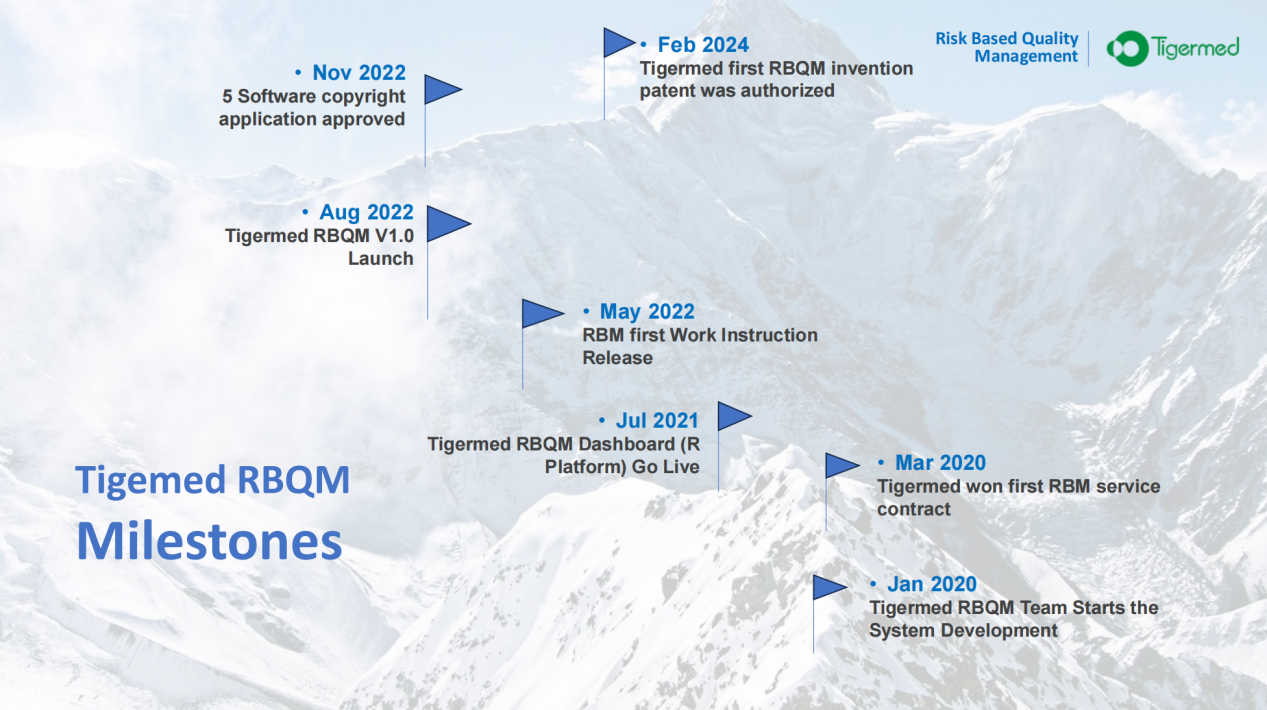
The
Tigermed RBQM team embarked on a major innovation project to develop a system
platform. After more than three years of exploration and construction, Tigermed
was authorized with its first RBQM invention patent. This patent provides
direct benefits to the quality, efficiency, and speed of clinical trials. At
the organizational level, Tigermed now offers a service portfolio that includes
modules for risk assessment and management, centralized monitoring, and
adaptive monitoring. It has also developed solutions and supporting systems
that are applicable to multi-regional clinical trials all over the world.

The Tigermed's RBQM system is a
digital-driven and data analysis platform. The platform provides
computerized system validation and features customizable clinical data analysis
with visualization capabilities. Its main functionality includes centralized monitoring, analyzing clinical
and operational data to identify outliers, risk trends, and data anomalies.
Also, the RBQM platform encompasses risk assessment and monitoring functions
such as key risk indicators and study metrics, along with risk detection
features like data quality assessment and data visualization.
Drug R&D is evolving to an
intelligent stage, and digital innovation has become the most important factor
in advancing drug R&D. Tigermed RBQM team will take this patent acquisition
as an opportunity to enhance technology innovation and to promote the
application of patent achievements, also set up SOPs internally from the
organizational level and clinical trial level, drive the RBQM business with a
standardized digital technology platform with high quality and efficiency, and
illuminate the road to the future of new drug development with innovative
tools.