Our Company
Culture & Values
Leadership
Global Locations
Corporate Compliance
Subsidiaries & Joint Ventures
Diversity & Inclusion
20th Anniversary
Medicinal Chemistry
Compound Screening
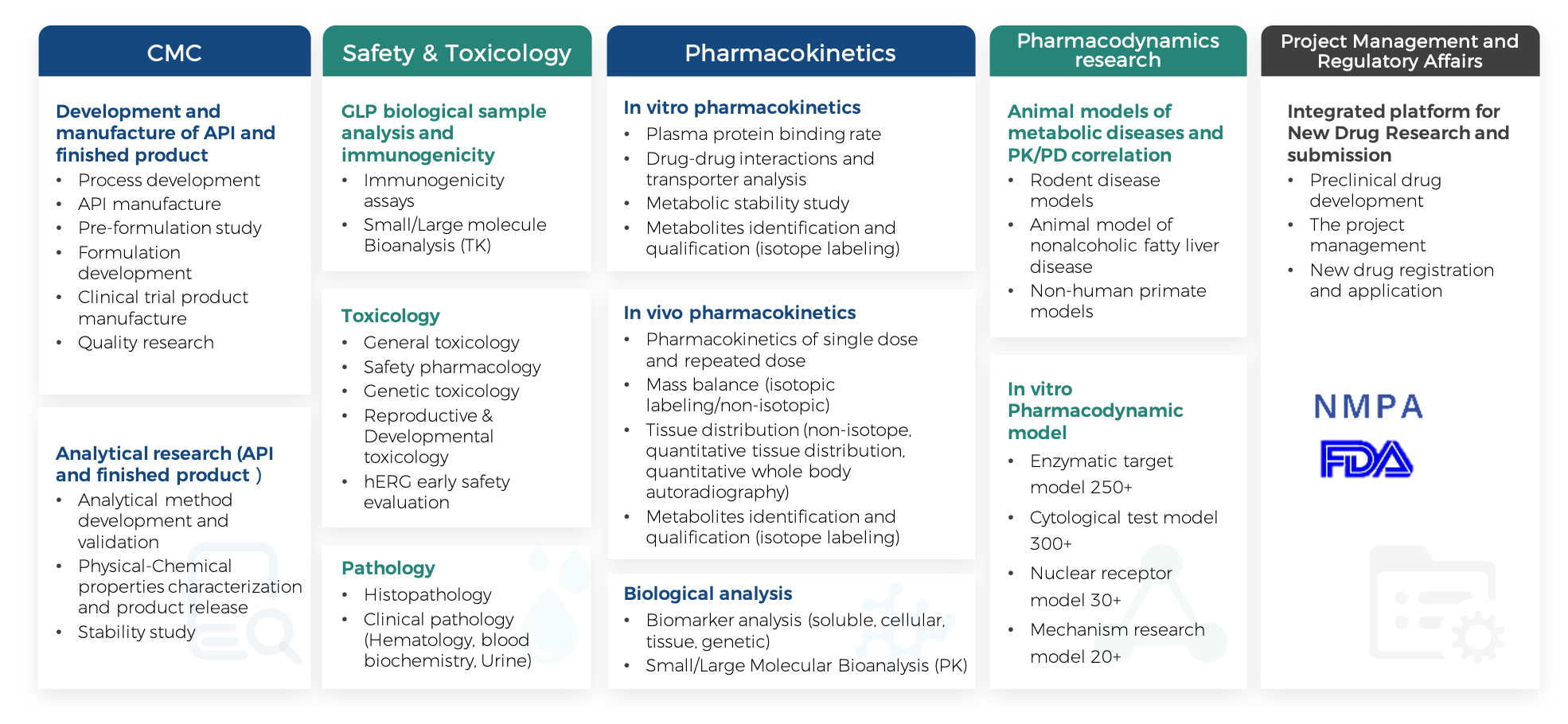
DMPK
Safety & Toxicology
Bioanalytical
CMC
Medical Writing
Clinical Monitoring
Regulatory Affairs
Data Management and Statistical Analysis
Clinical Development Strategy
Decentralized Clinical Trials (DCTs)
Site Management (SMO)
Medical Device / IVD
Multi-region Clinical Trial (MRCT)
Vaccine Clinical Trial